Revolutionising NATs Production: A Scalable and Sustainable Approach
Unlocking the full potential of nucleic acid therapeutics (NATs) has always been hindered by manufacturing challenges. Traditional chemical synthesis methods face limitations in scalability and sustainability, prompting the need for innovative solutions. This project, developed at the University of Manchester, brings an innovative solution ready to licence.
Challenging the Norms
Nucleic acid therapeutics (NATs) are crucial for selectively modulating the production of disease-related proteins. However, existing chemical synthesis methods are not conducive to large-scale applications, presenting several challenges:
- Solid supports and chemical synthesis methodologies limit production to small batches.
- Extensive washing steps and chromatographic purification necessitate the use of unsustainable volumes of acetonitrile.
- Currently marketed therapies suffer from low yields, modest purities, and complex mixtures of stereoisomers.
There’s a pressing need for a manufacturing paradigm shift, one that overcomes these limitations and unleashes the full potential of NATs.
The Technology
Researchers at the University of Manchester have pioneered a one-pot enzyme cascade for scalable oligonucleotide synthesis. This innovative methodology utilises DNA polymerases, NTP monomers, and an endonuclease to amplify a self-priming template in a repetitive process. Setting it apart from other methods, our cascade doesn’t require stoichiometric primers, ensuring unscarred products without complicating the purification process.
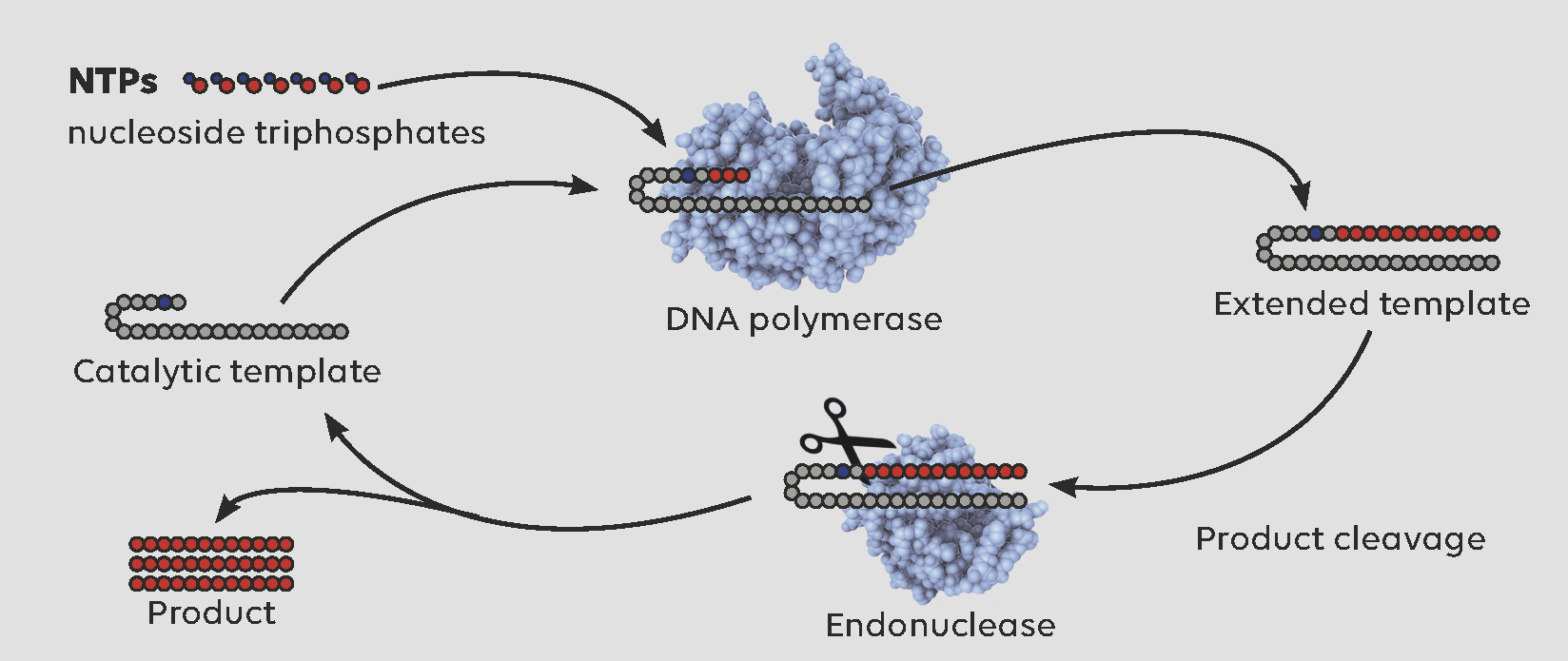
Modified image, original source: https://www.science.org/doi/10.1126/science.add5892
Key Benefits:
This revolutionary technology comes with a host of advantages:
- Operates in solution-phase under isothermal conditions, fitting seamlessly into conventional batch reactors.
- Significantly reduces raw material requirements, enhancing scalability and sustainability.
Capable of synthesising a diverse range of oligonucleotide sequences and modifications. - A one-pot methodology replaces iterative rounds of chain extension, oxidation, capping, and deprotection, streamlining the process.
- Products are synthesised with increased yield and purity, eliminating the need for solvent-based purification methodologies.
Licensing Opportunity:
This technology is available for licensing on a non-exclusive basis across various fields. In the therapeutic oligos domain, the University offers licences for research purposes or manufacturing products for clinical development/commercialisation. Please contact the Project Manager for more information on licensing this innovation.
References:
Moody, E. R. et al. “An enzyme cascade enables production of therapeutic oligonucleotides in a single operation.” Science 380, 1150-1154 (2023). https://www.science.org/doi/10.1126/science.add5892




