This is Licence-Ready
caADAMTS13 is a preclinical-stage biologic therapy that dissolves blood clots in acute ischemic stroke. The therapy could also be applied in other blood clotting diseases.
Acute ischemic stroke is one of the leading causes of death and disability around the world. It is caused by a blood clot that blocks the flow of blood to the brain, leading to neuronal damage. Current treatments focus on removing the clot using thrombolytic therapies or a form of surgery called a mechanical thrombectomy.
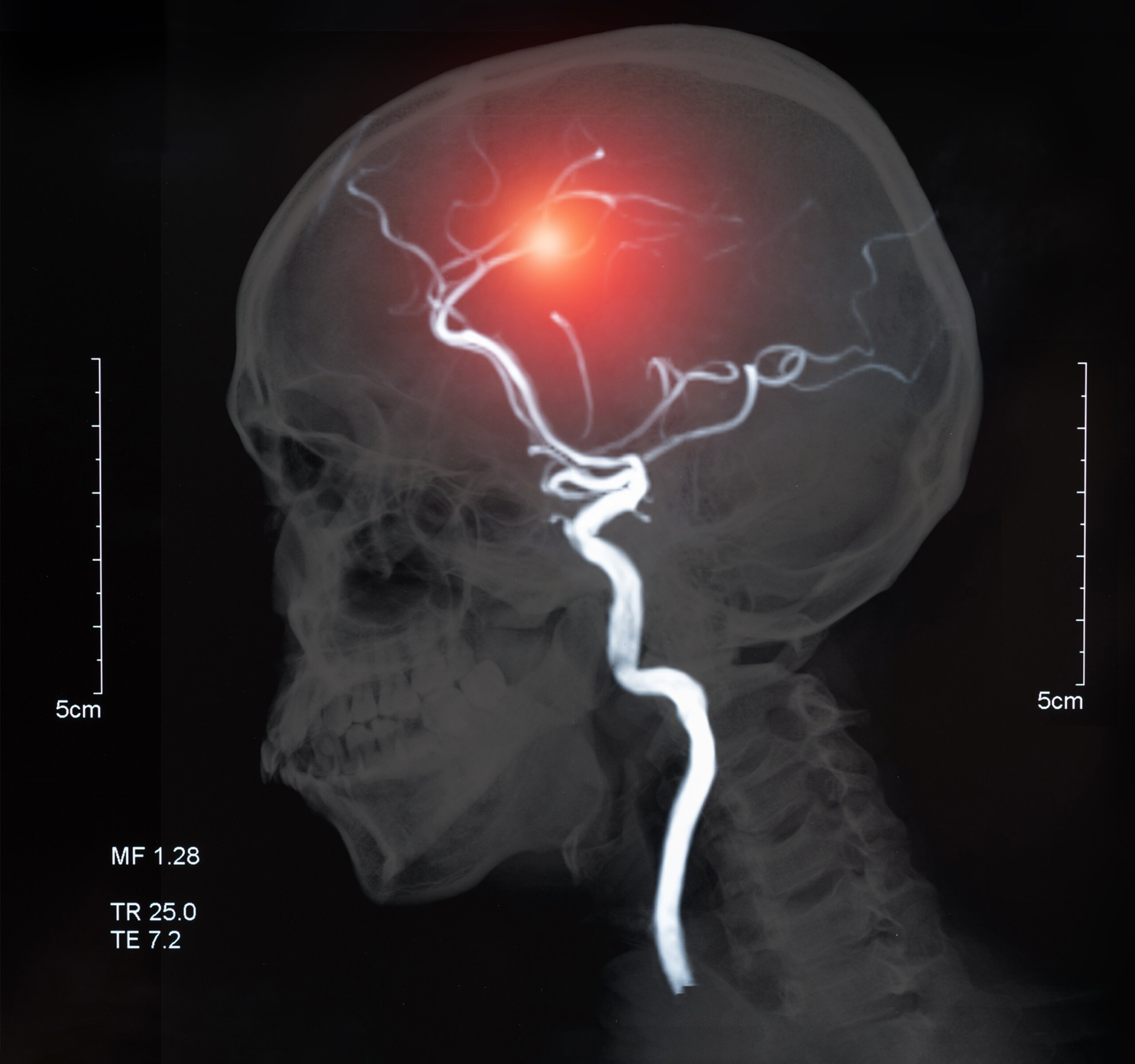
MRI of the blood vessels in the brain showing ischemic stroke
The current gold-standard in thrombolytic therapy – tissue plasminogen activator (t-PA) – can only be administered to patients who present to hospital within 4.5 hours of stroke onset. Where treatment is delayed, the potential benefits of t-PA treatment are outweighed by the increased risk of an intracerebral haemorrhage. Furthermore, 50% of patients who receive t-PA do not respond to treatment and go on to require a mechanical thrombectomy. There is an urgent need for new thrombolytic therapies.
A team of researchers at The University of Manchester, led by Prof. Stuart Allan and Dr. Kieron South, have developed a new clot-busting thrombolytic therapy called caADAMTS13. caADAMT13 is a constitutively active variant of the enzyme ADAMTS13. ADAMTS13 is known to have thrombolytic activity, but development of wild-type protein as a therapy has been limited as the dose required to elicit therapeutic benefit in patients is too high. caADAMTS13 is five-times more active than the wild-type protein and thus the required therapeutic dose is lower. The variant has been shown to be effective in clearing blot clots and improving blood flow, in two murine models of ischemic stroke (South et al., 2022).
Technology advantages:
- Reduced risk of haemorrhage compared to current standard of care (e., t-PA)
- Longer potential therapeutic window, meaning patients who present to hospital over 4.5 hours after stroke onset could receive thrombolytic therapy
- caADAMTS13 is active against an increased range of clot types (compared to t-PA), with potential to be effective in a greater number of patients
- Potential to be developed as a multi-indication therapy
A composition of matter patent application covering ADAMTS13 variants with increased catalytic activity has been filed by The University of Manchester (WO2022/175666).
The Innovation Factory is now seeking a licensee to drive forward preclinical and clinical development of this preclinical-stage biologic programme in acute ischemic stroke and secondary indications. Please get in touch with the project managers for more information.
References:
Kieron South, Ohud Saleh, Eloise Lemarchand, Graham Coutts, Craig J. Smith, Ingo Schiessl, Stuart M. Allan; Robust thrombolytic and anti-inflammatory action of a constitutively active ADAMTS13 variant in murine stroke models. Blood 2022; 139 (10): 1575–1587. doi: https://doi.org/10.1182/blood.2021012787