ManTRa Diagnostics has developed tumour-site-specific gene expression signatures to assess hypoxia which has the potential to deliver major advances in personalised cancer medicine
Solid tumours have different amounts of oxygen and low levels (hypoxia) are associated with resistance to treatment and a poor prognosis. Around a half of solid tumours will be hypoxic, which reduces the efficacy of surgery, radiotherapy, and many chemotherapeutic agents. Conversely, there is likely to be an enriched population with low tumour hypoxia with increased efficacy.
ManTRa Diagnostics has developed tumour-site-specific gene expression signatures to assess hypoxia which has the potential to deliver major advances in personalised cancer medicine. The signatures can be used as the basis for a Companion Diagnostic (CDx) to stratify patients for treatment and as a mechanism to direct therapy choices.
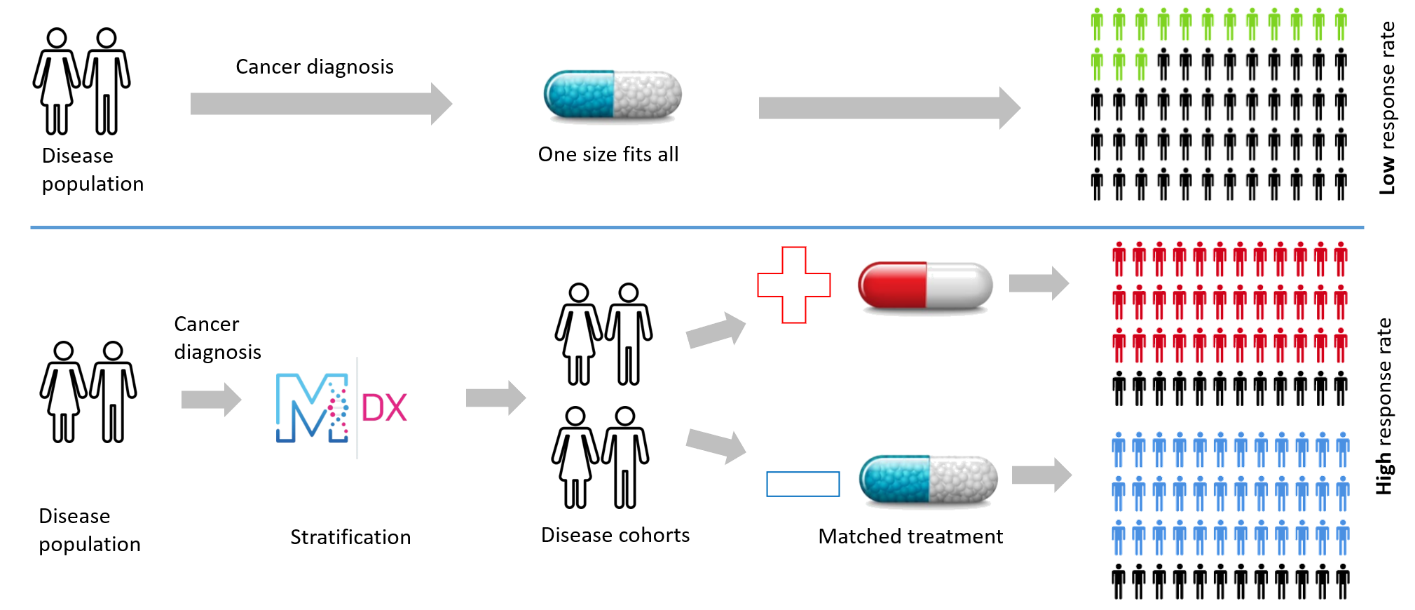
To date hypoxia related gene signatures have been identified and validated in Head and Neck, Prostate, Sarcoma and Bladder Cancers, with each signature containing 20-30 genes. Throughout these studies, use of the ManTRa Diagnostics method as a diagnostic, prognostic and prediction tool has been demonstrated as well as its robustness and reproducibility. The head & neck signature is currently in Phase III clinical trials and other signatures in clinical trial design and validation. The signatures are applicable to quantitative Polymerase Chain Reaction (qPCR) technology but are suitable for transfer to Next Generation Sequencing (NGS).
Value Proposition
ManTRa Diagnostics products have the potential to have wide-reaching benefits:
• Pharmaceutical companies will have fewer failed trials, increased market size and decreased time for approval.
• Patients will have personalised treatments and improved outcomes.
• The healthcare system will have more cost-effective cancer treatments.
Market
The failure of new drugs in late phase clinical trials costs the pharmaceutical industry millions. Over 97% of drugs do not progress to clinical use, which contributes to the $2.6Bn cost of developing a new therapeutic. The limitation of trials in unselected patients is highlighted by increasing efforts to discover, validate and use biomarkers.
The global oncology biomarkers market size was estimated at $10.89Bn in 2016 and is expected to reach $19.46Bn by 2021 growing at a Compound Annual Growth Rate (CAGR) of 12.3%1. The Companion Diagnostics market is expected to grow from $2.55Bn in 2017 to $8.12Bn by 2023, a CAGR of 21.3%1.
• By therapeutic area, oncology is anticipated to account for the largest share and the highest growth rate from 2017 to 2023 (26.2% CAGR).
• By Technology, PCR and NGS represent approximately 80% CDx market.



